Atomic Structure
Question 1
Write the electronic configuration for: C, N3-, Fe2+, Cu, Cu+, I and Pb.
Question 2.
Put the following atoms/ions in order of decreasing size. Explain your answer. The atoms/ions are: Na+, Al3+, Ne, Ar, K+, N3-.
Question 3:
Draw a graph representing all the ionisation energies possible for the Al atom.
Bonding
Question 1
Explain the trends in boiling points for the hydrogen halides.
Question 2
Draw the following molecules/ions: BH2–, NI3, ClF4, SF5–, BO33-, O32+, IO2+, BBr32-
Energetics
Question 1
On strong heating, calcium carbonate decomposes to calcium oxide and carbon dioxide:
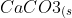}&space;\rightarrow&space;CaO_{(s)}&space;+&space;CO2_{(g)})
Owing to the conditions under which the reaction occurs, it is not possible to measure the enthalpy change directly.
An indirect method employs the enthalpy changes when calcium carbonate and calcium oxide are neutralized with hydrochloric acid.
i) Write the equation for the reaction of calcium carbonate with hydrochloric acid. State symbols are not required. [ΔH1 is the enthalpy change for this reaction]
ii) The reaction of calcium oxide with hydrochloric acid is
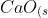}&space;+&space;2HCl_{(aq)}&space;\rightarrow&space;CaCl2_{(aq)}&space;+&space;H2O_{(l)}&space;\Delta&space;H2)
Use the equations in parts (i) and (ii) to complete the Hess’s Law cycle below to show how you could calculate the enthalpy change for the decomposition of CaCO3. Label the arrows in your cycle.
Question 2
Calculate ΔH and ΔS for the following reaction:
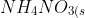}&space;+&space;H_2O_{(l)}&space;\rightarrow&space;NH_4^+_{(aq)}+NO_{(3)}-&space;_{(aq)})
Use the results of this calculation to determine the value of ΔGo for this reaction at 25o C, and explain why NH4NO3 spontaneously dissolves in water at room temperature.
Compound Hfo(kJ mol-1) S(J mol-1 K-1)
NH4NO3(s) -365.56 151.08
NH4+ (aq) -132.51 113.4
NO3– (aq) -205.0 146.4
Equilibria
Kc
Question 1
Esters are a useful group of compounds due to their distinctive smells. One example
of an ester is ethyl ethanoate, its formation is shown below.
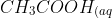}&space;+&space;C_2H_5OH_{(aq)}&space;\rightarrow&space;CH_3COO&space;C_2H_5_{(aq)}&space;+&space;H_2O_{(l)})
a) Systems like this are described as being a ‘dynamic equilibrium’. Explain
the term ‘dynamic equilibrium’
b) Write down the expression for the equilibrium constant, Kc, for this reaction.
c) Calculate the value of Kc for this reaction given the equilibrium concentrations below.
[CH3COOH] = 0.08 moldm-3, [C2H5OH] = 0.08 moldm-3, [CH3COO C2H5] = 0.25 moldm-3, [H2O] = 0.1 moldm-3
d) Concentrated sulphuric acid is added to the reaction mixture as it removes water molecules. What effect would this have on the equilibrium position of this system?
Question 2
A mixture of 1.441 g of H2 and 70.24 g of Br2 is heated in a 2.00-L vessel at 700 K. These substances react as follows.
}+Br_2_{(g)}\rightarrow&space;2HBr_{(g)})
At equilibrium, the vessel is found to contain 0.627g of H2. Calculate their equilibrium concentrations and Kc.
Kp
Question 1
Ammonium iodide dissociates reversibly to ammonia and hydrogen iodide.
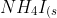}\rightleftharpoons&space;NH_3_{(g)}+HI_{(g)})
At 400oC,Kp=0.215. Calculate the partial pressure of ammonia at equilibrium when a sufficient quantity of ammonium iodide is heated to 400oC.
Question 2
For the reaction
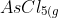}\rightleftharpoons&space;AsCl_3_{(g)}+Cl_2{(g)})
at 550 K, the equilibrium constant (Kp) is 9.81kPa. Suppose that 3.150 g AsCl5 is placed in an evacuated 600 ml bulb, which is then heated to 550K.
What is the partial pressure of AsCl5 at equilibrium? (For this question you would need to use Pv=nRT and in the ICE table, the pressure is used instead of moles)
